Is Matter Around Us Pure
✅ “Here is Complete notes on Is Matter Around Us Pure – definition, properties, states of matter, evaporation, sublimation, and key science concepts explained in simple language.”
Table of Contents:
-
What is matter?
- definition
- Classification of Ancient Indian Philosophers
- Classification of modern scientists
-
Physical properties of matter
- Matter is made up of particles
- Particles of matter are very small
- There is a space between particles of matter
- Particles of matter are constantly moving
- Particles of matter attract each other
-
States of matter
- Solid State
- Properties: Fixed size, fixed volume, incompressible
- Reason: Strong attraction force and low kinetic energy between particles
- Liquid State
- Properties: Fixed volume, indeterminate shape, somewhat compressible
- Reason: The attraction force between the particles is less than that of the solid and the kinetic energy is high.
- Gaseous State
- Properties: Indeterminate shape, indeterminate volume, highly compressible
- Reason: weak attraction force and high kinetic energy between particles
- Solid State
-
Can a substance change its state?
- Effect of Temperature
- Melting Point: The temperature at which a solid melts to form a liquid.
- Boiling point: The temperature at which a liquid boils to form a gas.
- Sublimation: The conversion of a solid directly into a gas or a gas directly into a solid. Examples: camphor, ammonium chloride.
- Effect of Pressure
- Gases can be converted into liquids by pressing.
- Effect of Temperature
-
Evaporation
- Definition: The gradual transformation of particles on the surface of a liquid into a gas.
- Factors Affecting Evaporation:
- Surface Area
- Temperature
- Humidity
- Wind Speed
- Why does evaporation cause cooling?
-
Condensation
- Definition: The conversion of a gas into a liquid.
-
Some Important Definitions of Is Matter Around Us Pure
- Diffusion
- Density
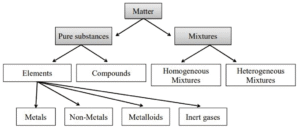
Detailed Notes:
2. What is Substance?
- Definition: Is Matter Around Us Pure? Matter is defined as any object that occupies space and has mass (weight). Everything around us—air, water, stones, plants, animals—is made up of matter. Understanding Is Matter Around Us Pure helps us learn about the nature and composition of these substances.
- Classification of Ancient Indian Philosophers: Ancient Indian philosophers classified matter into five basic elements (“Pancha Tattva”): earth, water, fire, air, and space.
- Classification of Modern Scientists: Modern scientists have classified matter on two main bases:
- On the basis of physical properties: solids, liquids and gases.
- On the basis of chemical nature: elements, compounds and mixtures (we will read this in the next chapters).
2.Physical properties of matter
✅ Is Matter Around Us Pure? Yes, because matter is made up of small particles, and these particles have some special properties that help us determine the purity of matter.
- Matter is made up of particles: At first it was believed that matter is continuous, but later experiments showed that matter is actually made up of very small particles. For example, when sugar or salt is dissolved in water, they spread evenly throughout the water, which indicates that water is also made up of particles and sugar/salt particles get incorporated into the spaces between them.
- Particles of matter are very small: Particles of matter are so small that we cannot see them with our naked eyes. There are billions of particles present in a crystal. When some crystals of potassium permanganate are dissolved in water, its color spreads throughout the water, and even after repeated dilution, the color becomes lighter, but does not disappear completely. This shows that even a small crystal of potassium permanganate contains millions of tiny particles.
- There is a space between particles of matter: when we dissolve sugar or salt in water, they do not disappear, but become incorporated into the spaces that exist between the particles of water. This shows that there is empty space between particles of matter.
- Particles of matter are constantly moving: Particles of matter do not remain stationary, but move continuously. It is the highest in gases, less in liquids and lowest in solids (vibrating only). When the incense stick is lit, its fragrance spreads throughout the room, this is due to the movement of the particles. This is called diffusion. The spontaneous motion of particles from a region of high concentration to a region of low concentration is called diffusion.
- Particles of matter attract each other: There is an attraction force between the particles of matter that keeps them bound together. This force is different in different substances. This force is highest in solids, due to which they have a definite shape. Liquids have less force, so they can flow and do not have a definite shape. In gases, this force is very low, so their particles continue to rotate freely.
Particle Theory – Is Matter Around Us Pure?
3. States of Matter:
Matter is mainly found in three physical states: solid, liquid, and gas.
- Solid State:
- Properties:
- Fixed Size: These have a fixed shape and cannot be easily changed.
- Fixed Volume: They have a fixed volume and occupy the same amount of space when placed in any container.
- Incompressible: They are difficult to press because their particles are arranged very closely and there is very little space between them.
- Reason: In solids, the particles are arranged very closely and there is a strong force of attraction between them. The kinetic energy of particles is very low, so they can only vibrate around their mean position.
- Examples: stone, wood, iron, ice.
- Properties:
- Liquid State:
- Properties:
- Indeterminate Shape: They do not have a definite shape. They take the shape of the vessel in which they are poured.
- Fixed Volume: These have a fixed volume.
- Somewhat compressible: They can be pressed slightly as compared to solids because they have more spaces between particles than solids.
- Reason: Particles in liquids are arranged farther apart than solids and the force of attraction between them is less than that of solids. The kinetic energy of particles is higher than solids, so they can drift around.
- Examples: water, milk, oil.
- Properties:
- Gaseous State:
- Properties:
- Indeterminate Shape: They do not have a definite shape.
- Indeterminate Volume: They do not have a fixed volume. They occupy the entire volume of the vessel in which they are kept.
- Highly compressible: They can be easily compressed because there is a lot of space between their particles.
- Reason: The particles in gases are arranged very far apart and the attraction force between them is very low. The kinetic energy of particles is very high, so they freely rotate rapidly in all directions.
- Examples: Air, oxygen, carbon dioxide.
- Properties:
4. Can a substance change its state?
Yes, a substance can change its state by changing temperature and pressure.
- Effect of Temperature:
- Melting Point: The fixed temperature at which a solid melts and converts into a liquid state is called its melting point. The melting point of a solid depends on the strength of the attraction force between its particles. The melting point of ice is 0°C (273.15 K).
- Boiling Point: The fixed temperature at which a liquid boils and converts into a gaseous state is called its boiling point. The boiling point of water is 100°C (373.15 K).
- Sublimation: Some solids convert directly into the gaseous state without changing to the liquid state on heating, and the gas directly converts into the solid state on cooling. This process is called sublimation.
- Examples: Camphor, Ammonium Chloride, Naphthalene.
- Effect of Pressure:
- Changes in pressure can also cause changes in the state of matter, particularly in the case of gases.
- By increasing the pressure on gases and lowering the temperature, they can be converted into liquids. For example, LPG (LPG) and compressed natural gas (CNG) are liquefied at high pressure and filled into cylinders.
5. Evaporation & Is Matter Around Us Pure
- Evaporation is the process of gradual conversion of particles of a liquid into gaseous state at any temperature. It is different from boiling because boiling occurs simultaneously throughout the fluid and occurs at a certain temperature.
- Factors Affecting Evaporation:
- Surface Area: The rate of evaporation increases as the surface area increases. For example, clothes are spread out and poured in to dry.
- Temperature: The rate of evaporation increases as the temperature increases as more particles get enough energy to convert into a gaseous state.
- Humidity: The amount of water vapour present in the air is called humidity. If there is already more water vapour present in the air, the rate of evaporation decreases. Evaporation occurs faster in dry air.
- Wind Speed: The rate of evaporation increases when there is a strong wind because the air blows away the particles of water vapour from the surface, causing more evaporation.
- Why does evaporation cause cooling? During evaporation, the particles on the surface of the fluid change into a gaseous state by gaining energy. They take this energy from the surrounding fluid or surface, due to which the surrounding temperature drops and we feel cool. For example, the evaporation of sweat in summer cools us down.
6. Condensation
- Definition: The process of change from gaseous state to liquid state is called condensation. It is the opposite process of evaporation. When water vapour comes in contact with the cold surface, it loses its energy and turns into a fluid. For example, water droplets accumulating on the outer surface of a cold water bottle.
7. Some Important Definitions of Is Matter Around Us Pure:
- Diffusion: The spontaneous mixing of particles of two or more substances into each other is called diffusion. Diffusion occurs most rapidly in gases, followed by liquids and slowest in solids. The rate of diffusion increases as the temperature increases because the kinetic energy of the particles increases.
- Density: The mass of a substance in its unit volume is called its density. Density = Mass / Mass Volume (ρ=Vm). The density of solids is usually higher than that of liquids and gases.
This chapter provides a basic understanding of matter around us. In the following chapters, we shall learn more about the chemical properties of substances and their classification.
Key things to remember:
- Matter is made up of particles.
- Particles of matter are very small and there is space between them.
- Particles of matter are constantly moving and attract each other.
- There are three main states of matter: solid, liquid, and gas.
- The state of a substance can be changed by changing the temperature and pressure.
- Evaporation is a superficial process that provides cooling.
Diffusion is the spontaneous mixing of particles of substances.